 GUESS how thoughtless I am!!! Almost forgot to post this entry of our DEAR uncle atmosphere!! Heard this news from the news roundup couple days ago, since the pollution of the atmosphere has been reducing... the health of our atmosphere is experiencing an obvious upturn. The scientists discovered that the split found in the atmosphere in the 20th century is now pretty stable and the size of it is diminishing naturally. There is a prospect of the atmosphere will be completely self-recovered in 60 years!! Ehmmm wish I could be part of the audience to share this wonderful news by then :P The following are some information about atmospheric pollution and the atmosphere for your review... CHEERS :D
GUESS how thoughtless I am!!! Almost forgot to post this entry of our DEAR uncle atmosphere!! Heard this news from the news roundup couple days ago, since the pollution of the atmosphere has been reducing... the health of our atmosphere is experiencing an obvious upturn. The scientists discovered that the split found in the atmosphere in the 20th century is now pretty stable and the size of it is diminishing naturally. There is a prospect of the atmosphere will be completely self-recovered in 60 years!! Ehmmm wish I could be part of the audience to share this wonderful news by then :P The following are some information about atmospheric pollution and the atmosphere for your review... CHEERS :DCause of Pollution
Since the onset of the Industrial Revolution in the late 18th century, most manufacturing and power-generation concerns have obtained their supplies of energy through the combustion of fossil fuels. Such combustion processes promote the release of various pollutants into the atmosphere. General usage of the term 'air pollution' refers to the anthropogenic input of such pollutants into the troposphere. Pollutants in the troposphere usually have quite a short residence time since they tend to be washed out by rain, altered by chemical reaction or deposited on the ground within a short while of their input. Pollutants that are injected directly into the stratosphere are usually characterised by a much longer residence time since removal mechanisms are weaker due to the comparative lack of water in this layer of the atmosphere. Natural sources, including volcanic eruptions, seaspray and lightning also contribute large volumes of gases that are considered as pollutants.
Classification of Atmospheric Pollutants
Pollutants of the atmosphere may be classified according to their source or according to their chemical composition. Classification according to source is dependent on the site of formation of a pollutant: Primary pollutants are formed within ground-based sources (e.g. factories, cars, power plants, volcanoes, sea-spray) and released directly into the atmosphere. Secondary pollutants (e.g. acids, ozone), on the other hand, are formed in situ within the atmosphere as a result of the reaction of various primary pollutants with each other and with other constituents of the atmosphere.
Types of Atmospheric Pollutants
Various groups of pollutants may be identified according to chemical structure, and the following scheme should not be considered exclusive.
1. Suspended particulate matter (SPM): small solid particles such as dust, soot, ash, sand. These may be derived from combustion (soot, ash) or from natural sources (dust, sand). Particles smaller than 10ì in diameter, called PM10, can pose a health risk.
2. Volatile organic compounds (VOC): mainly hydrocarbons, derived from boilers and central heating units, mainly due to burning of coal. VOC can also escape from vehicle engines into the atmosphere. Such pollutants may interfere with other atmospheric processes leading to a formation of tropospheric ozone.
3. Oxides: this class of pollutants comprises carbon dioxide (CO2), carbon monoxide (CO), sulphur dioxide (SO2), nitrogen dioxide (NO2) and nitric oxide (NO). All of these are products of combustion. Carbon monoxide is a toxic gas released from vehicles, and its concentration in the atmosphere has increased with the rise in traffic density. Sulphur dioxide is derived from the sulphur present in most fossil fuels. Burning of these fuels oxidises the sulphur and converts it to sulphur dioxide. This gas can contribute to the formation of acid rain. Nitrogen oxides are mainly derived from combustion and contribute to the formation of acid rain.
4. Lead: the use of lead as an anti-knocking agent in petrol leads to the emission of this metal (as part of a chemical compound such as PbCl2) in automobile exhaust. Inhalation of lead compounds can produce serious effects on human health particularly in children.
5. Ozone (O3): although stratospheric ozone (as the ozone layer) is considered beneficial to humans, it nevertheless remains a health hazard. Ozone can form in the troposphere through photochemical reactions involving the breakdown of nitrogen dioxide. Since it is not directly emitted into the atmosphere from groundbased sources it therefore qualifies as a secondary pollutant. Some tropospheric ozone also accumulates through transfer from the stratosphere.
6. Radon: this gas is a natural product of the radioactive decay of uranium that occurs in rocks and soil. Since radon is radioactive, it can decay to form small electrically-charged particles than can bind to small particles of dust present in the air. When this dust is inhaled, these particles (which emit radiation) are introduced into therespiratory system.
Atmosphere
The present atmosphere of the Earth is probably not its original atmosphere. Our current atmosphere is what chemists would call an oxidizing atmosphere, while the original atmosphere was what chemists would call a reducing atmosphere. In particular, it probably did not contain oxygen.
Composition of the Atmosphere
The original atmosphere may have been similar to the composition of the solar nebula and close to the present composition of the Gas Giant planets, though this depends on the details of how the planets condensed from the solar nebula. That atmosphere was lost to space, and replaced by compounds outgassed from the crust or (in some more recent theories) much of the atmosphere may have come instead from the impacts of comets and other planetesimals rich in volatile materials.
The oxygen so characteristic of our atmosphere was almost all produced by plants (cyanobacteria or, more colloquially, blue-green algae). Thus, the present composition of the atmosphere is 79% nitrogen, 20% oxygen, and 1% other gases.
Layers of the Atmosphere
The atmosphere of the Earth may be divided into several distinct layers, as the following figure indicates.
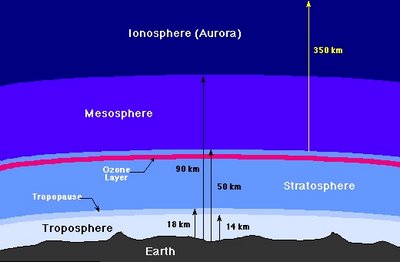
The Troposphere
The troposphere is where all weather takes place; it is the region of rising and falling packets of air. The air pressure at the top of the troposphere is only 10% of that at sea level (0.1 atmospheres). There is a thin buffer zone between the troposphere and the next layer called the tropopause.
The Stratosphere and Ozone Layer
Above the troposphere is the stratosphere, where air flow is mostly horizontal. The thin ozone layer in the upper stratosphere has a high concentration of ozone, a particularly reactive form of oxygen. This layer is primarily responsible for absorbing the ultraviolet radiation from the Sun. The formation of this layer is a delicate matter, since only when oxygen is produced in the atmosphere can an ozone layer form and prevent an intense flux of ultraviolet radiation from reaching the surface, where it is quite hazardous to the evolution of life. There is considerable recent concern that manmade flourocarbon compounds may be depleting the ozone layer, with dire future consequences for life on the Earth.
The Mesosphere and Ionosphere
Above the stratosphere is the mesosphere and above that is the ionosphere (or thermosphere), where many atoms are ionized (have gained or lost electrons so they have a net electrical charge). The ionosphere is very thin, but it is where aurora take place, and is also responsible for absorbing the most energetic photons from the Sun, and for reflecting radio waves, thereby making long-distance radio communication possible.
The structure of the ionosphere is strongly influenced by the charged particle wind from the Sun (solar wind), which is in turn governed by the level of Solar activity. One measure of the structure of the ionosphere is the free electron density, which is an indicator of the degree of ionization.






















